
CBGM Faculty - Dr. Shruti Sharma's Lab
IL-6 signaling | Barrier dysfunction | Angiogenesis | VEGF signaling | Diabetic retinopathy | Dry eye disease | Translational research

Shruti Sharma, MS, PhD
Associate Professor
Center for Biotechnology & Genomic Medicine
James and Jean Culver Vision Discovery Institute
Department of Ophthalmology
Medical College of Georgia at Augusta University
706-721-6522
Office: CA-4139 | Lab: CA-4132A
Jump to: Research Interests Projects Funding & Teaching Media Articles & Publications Graduate Students & Staff
Research Interests
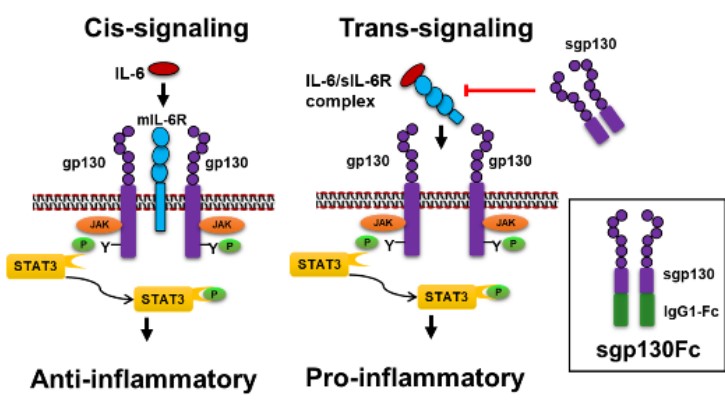
The Shruti Sharma Lab at Augusta University focuses on understanding the molecular mechanisms underlying ocular surface and retinal diseases, with particular emphasis on dry eye disease (DED) and diabetic retinopathy (DR). In DED, the lab utilizes both in vitro and in vivo models to investigate tear film instability, epithelial dysfunction, and chronic ocular surface inflammation. Tear fluid from patients is analyzed using advanced proteomic and glycomic approaches to identify disease-specific biomarkers and therapeutic targets. The lab has identified interleukin-6 (IL-6) trans-signaling as a novel therapeutic target in DR and investigates inflammatory and angiogenic pathways in endothelial and Müller glial cells, particularly the roles of IL-6 cis- and trans- balance and its effect on VEGF signaling. Preclinical studies utilizing sgp130Fc, a selective inhibitor of IL-6 trans-signaling, has shown decreased inflammation, oxidative stress, and retinal vascular damage.
Projects
IL-6 is a pleiotropic cytokine that possess both anti- and pro-inflammatory properties. Our lab found that inhibiting pro-inflammatory, IL-6 trans-signaling, attenuates inflammation, and helps prevent the vascular dysfunction in the retina that can lead to vision loss.
Our lab investigates the role of inflammation in DED, to find alternative therapeutic interventions. We center our work on understanding the mechanisms behind tear film instability and increased tear osmolarity, which are the major characteristics of DED.
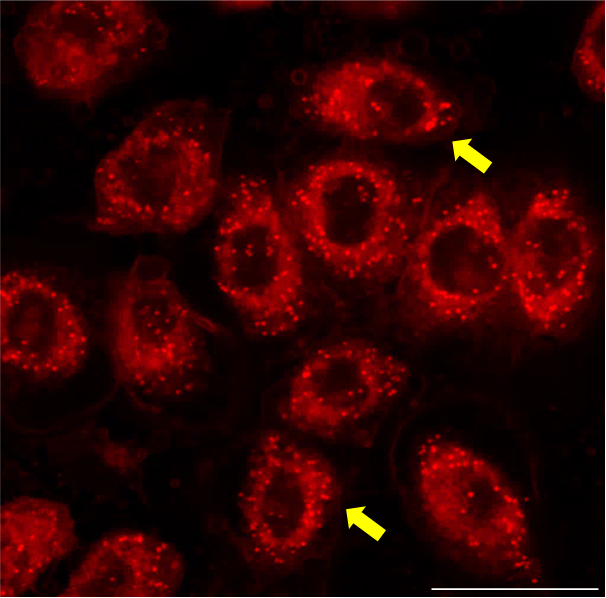
Nile red staining in hTCEpi cells
Our large collection of human tear samples allows us to identify DED tear biomarkers to further advance our search for potential therapeutic DED targets.
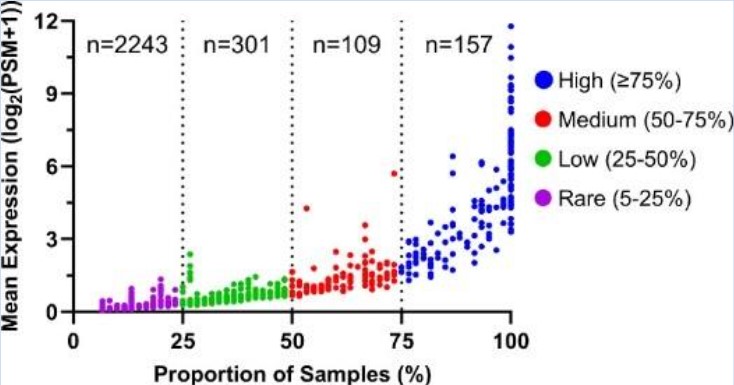
Proteins detected in human tear samples
Funded Grants & Teaching
Funding:
- NIH/NEI R01 grant: “Targeting Interleukin-6 cis-trans balance in Müller cell dysfunction”
- NIH/NEI R01 grant: “Sex-specific Influences on tear microRNAs in dry eye disease”
- Intramural Grant: Investigating Potential Treatments for Dry Eye Disease in Mouse Models
Teaching:
- Course Director, Translational Genomics and Proteomics (GNMD 8051)
- Course Director, Genomic Medicine Seminar (GNMD 8060)
- Course Director, Functional Genomics and Proteomics Using Animal Models (GNMD8052)
Selected Publications & Media Articles
- L-carnitine partially restores adherens junction integrity and promotes wound healing
in human corneal epithelial cells exposed to hyperosmolar stress.
Kontoh-Twumasi R, Aliste A, Scheid A, Glass J, Sharma A, Sharma S. Experimental Eye Research(2025) - Proteomic Alterations in Retinal Müller Glial Cells Lacking Interleukin-6 Receptor:
A Comprehensive Analysis.
Glass J, Robinson R, Edupuganti N, Altman J, Greenway G, Lee TJ, Zhi W, Sharma A, Sharma S. Investigative Ophthalmology & Visual Science(2024) - Role of Serine Protease Inhibitors A1 and A3 in Ocular Pathologies.
Kontoh-Twumasi R, Budkin S, Edupuganti N, Vashishtha A, Sharma S. Investigative Ophthalmology & Visual Science(2024) - Diabetic Müller-Glial-Cell-Specific Il6ra Knockout Mice Exhibit Accelerated Retinal
Functional Decline and Thinning of the Inner Nuclear Layer.
Glass J, Robinson RL, Greenway G, Jones G, Sharma S. Investigative Ophthalmology & Visual Science(2023) - Generation and characterization of a Müller-glial-cell-specific Il6ra knockout mouse
to delineate the effects of IL-6 trans-signaling in the retina.
Robinson R, Glass J, Sharma A, Sharma S. Scientific Reports (2022) - Interleukin-6 trans-signaling mediated regulation of paracellular permeability in
human retinal endothelial cells.
Glass J, Robinson R, Lee TJ, Sharma A, Sharma S; International Journal of Translational Medicine (2021) - Interleukin-6 trans-signaling: a pathway with therapeutic potential for diabetic retinopathy.
Sharma S; Frontiers in Physiology (2021) - RNA-Seq analysis reveals gene expression changes induced by IL-6 trans-signaling activation
in retinal endothelial cells.
Robinson R, Brown D, Churchwell L, Lee TJ, Kodeboyina SK, Bloom J, Sharma A, Sharma S; Cytokine(2021) - Diabetes induced alterations in murine proteome are mitigated by IL-6 trans-signaling
inhibition.
Robinson R, Youngblood H, Iyer H, Bloom J, Lee TJ, Chang L, Lukowski Z, Zhi W, Sharma A, Sharma S;Investigative Ophthalmology & Visual Science (2020) - Interleukin-6 trans-signaling inhibition prevents oxidative stress in a mouse model
of early diabetic retinopathy.
Robinson R, Srinivasan M, Shanmugan A, Ward A, Ganapathy V, Bloom J, Sharma A, Sharma S; Redox Biology (2020)
Media Articles
Graduate Students & Lab Staff


Richard Kontoh-Twumasi
- Graduate Research Assistant,
- Class of 2022
706-721-3404

Graduated Students
Rebekah Robinson
- MD/PhD Student
Project: IL-6/gp130 signaling in diabetic retinopathy

Arthur Miller, MD
- MD Student
- Medical College of Georgia (MCG)
Medical Residents & Students


Neel Edupuganti
- Medical Student (M4)
- Medical College of Georgia (MCG)
706-731-3404
Laboratory Staff





