
- Augusta University
- Centers & Institutes
- Vascular Biology Center
- Research Publications
Research Publications
Research Interests
The main goal of the laboratory is to analyze the mechanisms whereby metabolic disorders such as obesity, diabetes and lipodystrophy, lead to cardiovascular disease. More specifically, we are interested in determining the contribution of leptin, a hormone secreted by the adipose tissue, to vascular disease and hypertension. A major focus of the lab is to understand the sex-specificity of the mechanisms leading to cardiovascular disease in obesity and diabetes and to understand how metabolic disorders abrogate the protective effects of female sex hormones. All experiments are conducted in close collaboration with physicians and involve an integrative approach consisting of a wide range of physiological, pharmacological and molecular methods, and involving genetically engineered mouse models of disease.
Jump to: Research Projects Spotlight Publications Recent Publications
Research Projects
1. Contribution of the “leptin-adrenal-endothelial mineralocorticoid receptor axis” to cardiovascular disease in premenopausal women.
While women are initially view as protected from cardiovascular disease until menopause, compelling evidence indicates that obesity and high salt diet abrogate the protective effects of female sex hormones. One of the major goals of the laboratory is to test whether increases in leptin signaling could contribute to cardiovascular disease in premenopausal women. While combining the use of genetically engineered mouse models with in vitro experiments and studies in discarded human tissues, we have identified the adipokine leptin as a new direct regulator of aldosterone production by demonstrating that leptin acts in the adrenal glands to increase aldosterone synthase (CYP11B2) expression via calcium-dependent mechanisms and stimulate aldosterone production (Figure 1). We extended our findings by showing that leptin-mediated aldosterone production plays a major role in the development of cardiovascular disease in obese female by impairing endothelial function. These findings led to the proposal of the new concept that obesity induces hypertension via sex-specific mechanisms: leptin-mediated sympatho-activation in males and leptin-induced aldosterone production in females (Figure 2). More recently we demonstrated that the female sex hormone progesterone predisposes women to leptin- and aldosterone-induced vascular dysfunction and hypertension via upregulating endothelial mineralocorticoid receptor expression (Figure 3). Current projects aim at further understanding the origin(s) of the sex differences and the molecular mechanisms involved in MR regulation.
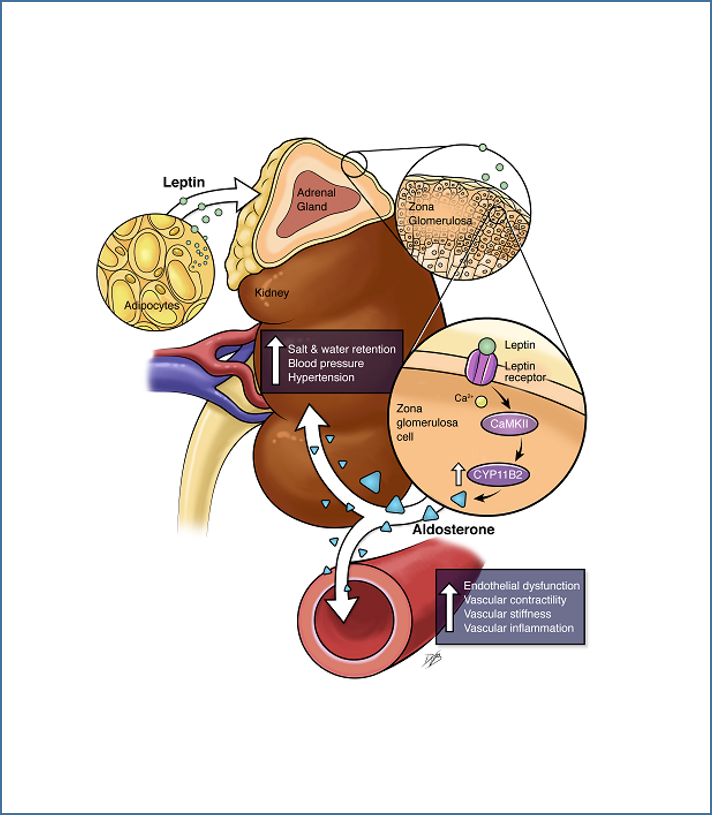
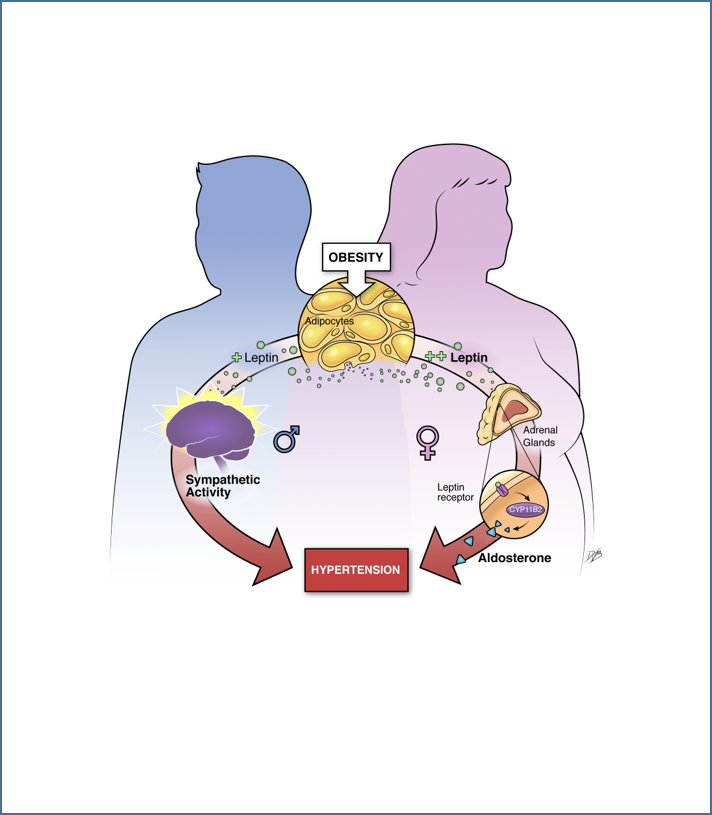
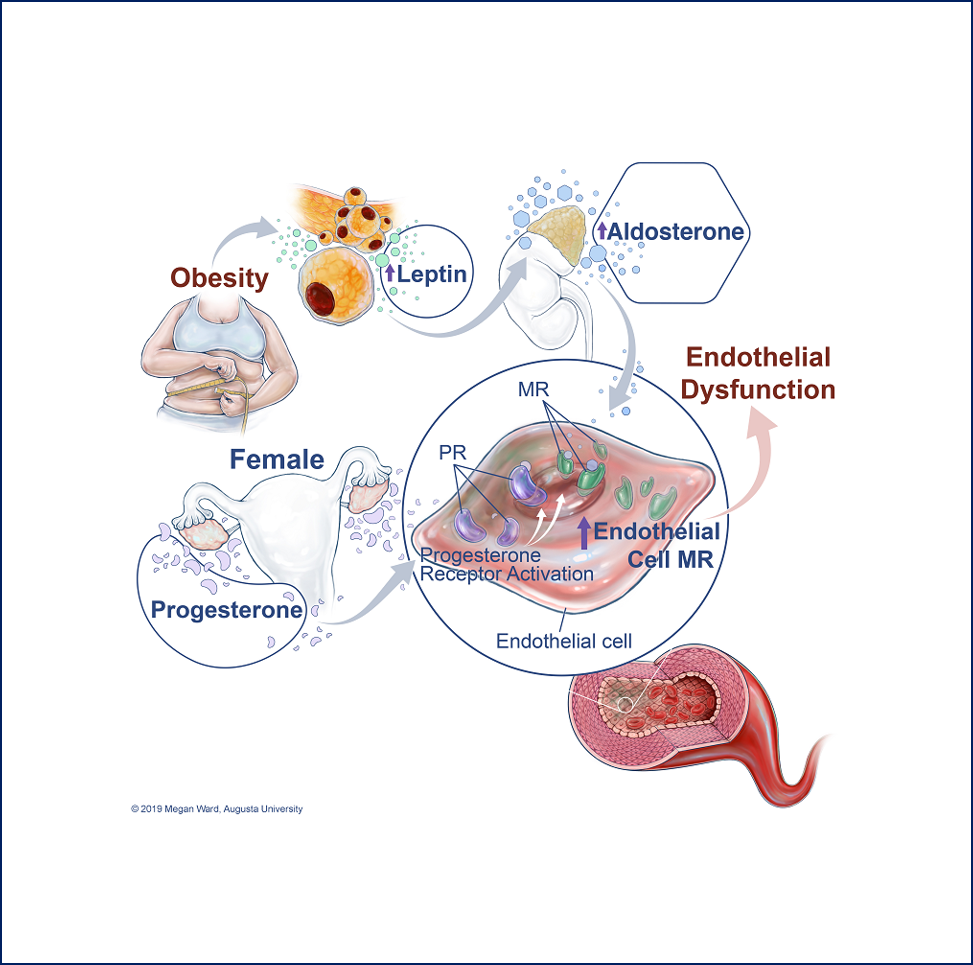
Figure 1. Huby, AC. Circulation. 2015 Figure 2. Huby, AC. Hypertension. 2016 Figure 3. Faulkner, JL. Hypertension. 2019
2. Leptin control of endothelial oxidative stress
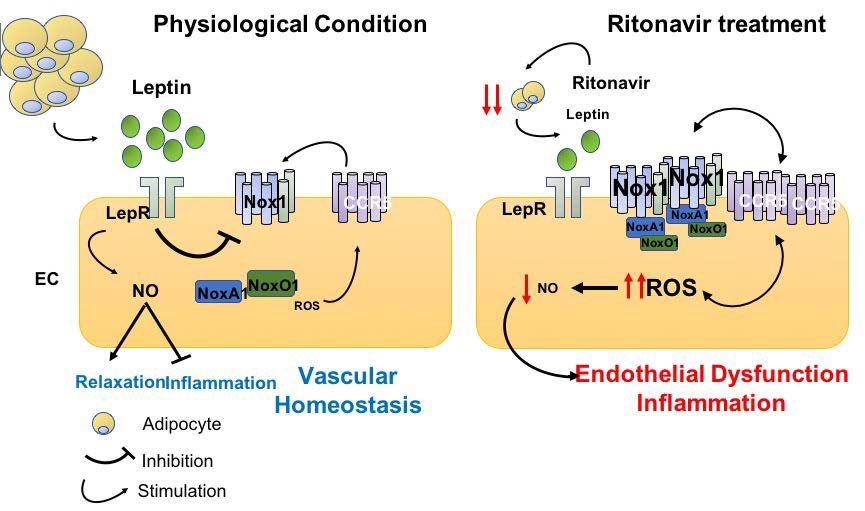
Conditions associated with reduced fat mass such as congenital generalized lipodystrophy, type I diabetes, and HIV-related lipodystrophy are a cause of reduced NO bioavailability and endothelial dysfunction. Based on the evidence that leptin regulates endothelium NO bioavailability in males, a question raised by the laboratory is whether reduced leptin levels consequent to low-fat mass contributes to endothelial dysfunction. Using a mouse model of congenital generalized lipodystrophy (mice deficient in Berardinelly Seip gene, Bscl2), but also with mouse model mimicking HIV-associated lipodystrophy (ritonavir treatment) we revealed that deficiency or insufficient leptin levels cause endothelial dysfunction by mechanisms involving reduced activation of endothelial leptin receptor. We showed that reduced endothelial leptin signaling decreases peroxisome proliferator-activated receptor-gamma (PPARg) expression and increases in NADPH oxidase NOX1, C-C chemokine receptor type 5 (CCR5) and vascular inflammation in male animals (Figure 4). Current investigations aim to understand (1) the sex specificity of these mechanisms, (2) the molecular links between leptin, PPARg, NOX1 and CCR5 and (3) the identity of the fat depot responsible for the positive effects of leptin on endothelial function.
3. Mechanisms of vascular disease and hypertension in HIV.
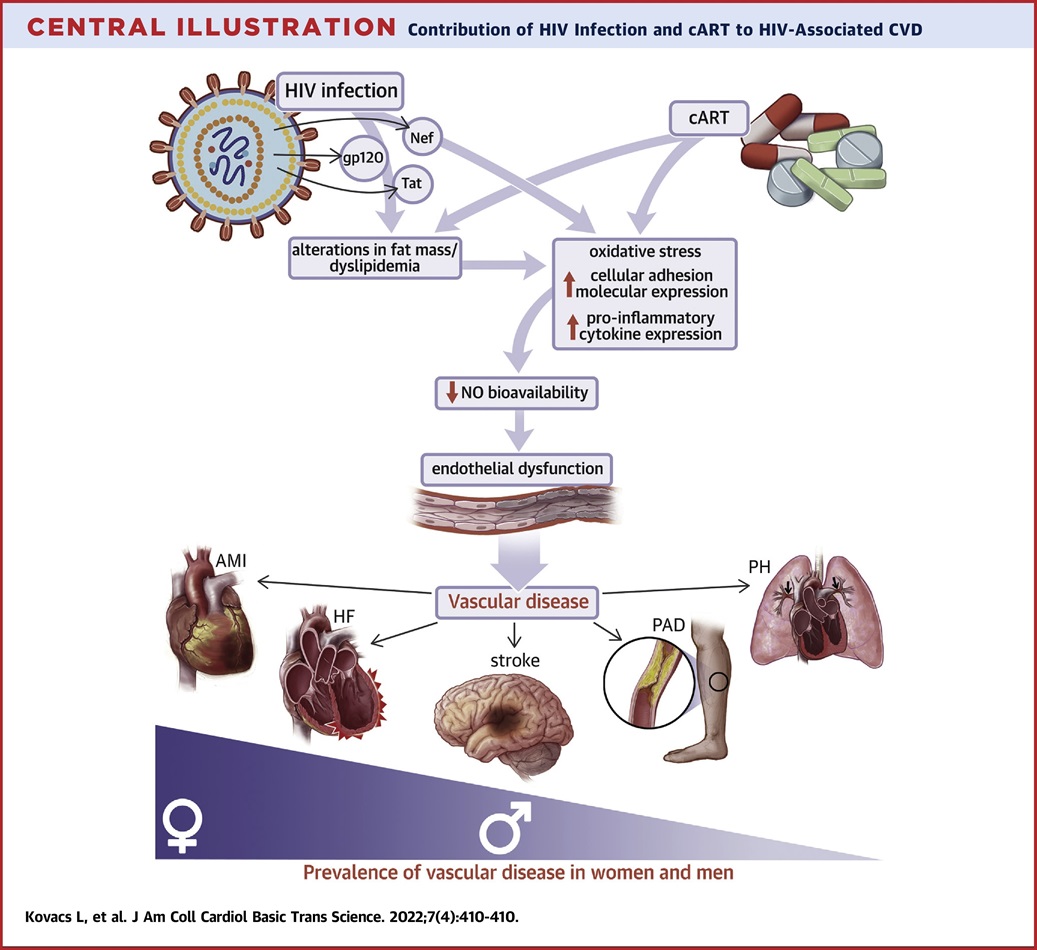
Thanks to the advent of combination antiretroviral therapy (cART), life expectancy has markedly increased in patients living with HIV, who now exhibit accelerated development of cardiovascular disease.
A major question raised is the contribution of repressed viral infection and cART to HIV-associated hypertension, endothelial dysfunction and atherosclerosis.
Using a transgenic mouse model mimicking repressed viral infection (Tg26 mouse), the laboratory is investigating the contribution of both HIV viral proteins and cART to endothelial dysfunction, hypertension and atherosclerosis (Figure 5).
As patients with HIV now present with obesity, an objective of the laboratory is also to understand the mechanisms whereby HIV viral proteins and cART affect metabolic function and energy expenditure.
Spotlight Publications

Huby, G. Antonova, J. Groenendyk, CE. Gomez-Sanchez, WB. Bollag, JA. Filosa, EJ. Belin de Chantemèle. The Adipocyte-Derived Hormone Leptin is a Direct Regulator of Aldosterone Secretion, Which Promotes Endothelial Dysfunction and Cardiac Fibrosis. (2015) Circulation. 132(22):2134-45
Huby AC, Otvos L, Belin de Chantemèle EJ. Leptin Induces Hypertension and Endothelial Dysfunction via Aldosterone-Dependent Mechanisms in Obese Female Mice. (2016). Hypertension. 67(5):1020-8
Faulkner JL, Harwood D, Bender L, Shrestha L, Brands MW, Morwitzer MJ, Kennard S, Antonova G, Belin de Chantemèle EJ. Lack of suppression of aldosterone production leads to salt sensitive hypertension in female but not male Balb/C mice. (2018) Hypertension, 72:1397-1406
Faulkner JL, Kennard S, Huby AC, Antonova G, Lu Q, Jaffe IZ, Patel VS, Fulton DJR, Belin de Chantemèle EJ. Progesterone predisposes females to obesity-associated leptin-mediated endothelial dysfunction via upregulating endothelial mineralocorticoid receptor expression. (2019) Hypertension, 74(3):678-686
Bruder-Nascimento T, Kennard S, Faulkner JL, Antonova G, Haigh S, Patel VS, Fulton DJR, Chen W, Belin de Chantemèle EJ. Leptin replacement therapy restores endothelial function, vascular adrenergic contractility and heart rate in a mouse model of congenital generalized lipodystrophy (CGL). (2019) Hypertension, 74(6):1399-1408. PMID: 31656096 PMCID: PMC6886673