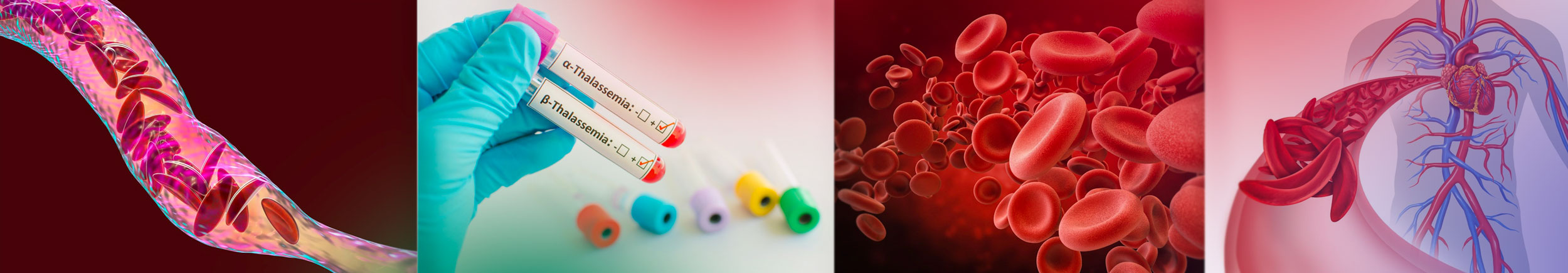
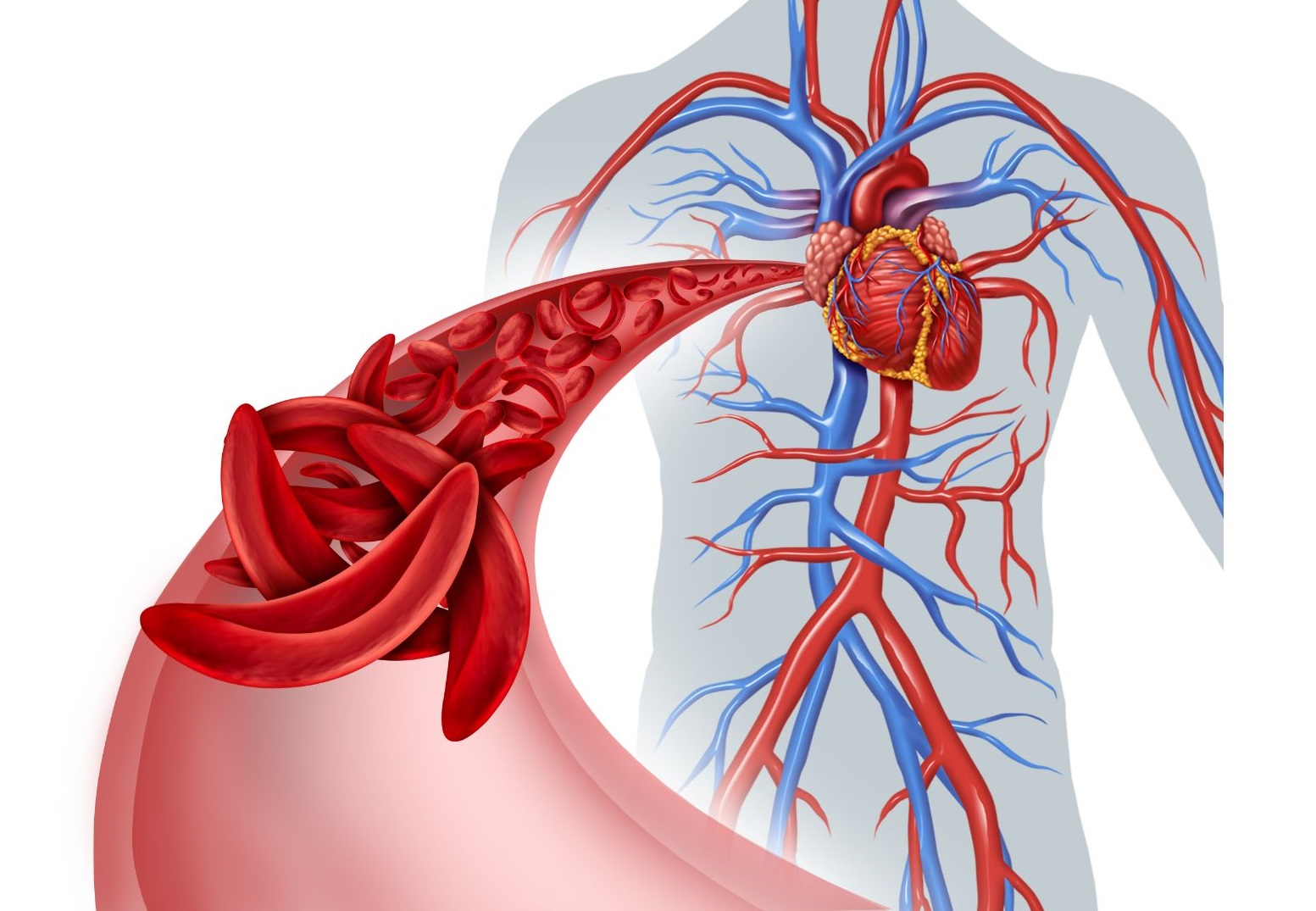
Woman becomes first in Augusta area to get bone marrow transplant to cure sickle cell disease
Alexis Jones-Heggs, was the first in the Augusta area to receive a bone-marrow transplant to try and cure her of sickle cell disease as part of a national clinical trial that involves the Sickle Cell Center at Augusta University.
Continue Reading