Jian-Kang Chen
 Jian-Kang Chen
Jian-Kang Chen
Associate Professor
Cellular Biology and Anatomy
Department of Medicine (secondary)
Office: CB2200, Research & Education Building
Lab: CB2615 and CB2102, Research & Education Building
Phone: 706.721.8424
Lab: 706.721.1618
jchen@augusta.edu
Members of the Chen Laboratory
Graduate Student: Desmond Moronge
Research Assistant: Caihong Dai, BS
Research Interests
- Autophagy and cell survival, growth, and proliferation
- Nephron growth and nephron damage
- Proximal tubular cell injury, cell death, and renal fibrosis
- Podocyte biology and proteinuric kidney disease
Research Approaches
- Molecular Biology approaches (e.g., immunoprecipitation, western blotting, RT-PCR, Northern blotting, Southern blotting, recombinant DNA cloning, gene targeting, and kinase activity assays).
- Cell cultures (both primary and cell lines as well as bacterial culture).
- Transgenic, conventional gene knockout, conditional Cre-Lox techniques, and gene knockin mouse models.
- Establishing mouse models of preclinical diseases (e.g. unilateral nephrectomy, unilateral ureteral obstruction mouse model, ischemia/reperfusion-induced acute kidney injury model, and mouse models of diabetic nephropathy).
- Cell Biology approaches including high-resolution microscopy (e.g., electron microscopy, confocal imaging, immunocytochemistry, immunofluorescent staining, immunohistochemistry, and flow cytometry analysis).
- Gene expression analyses (e.g., construction, transfection and expression of various plasmids including fluorescent reporter, cDNA microarray, and RNA-sequencing (RNA-seq) coupled with the Gene Set Enrichment Analysis of the whole-transcriptome).
Funding Support
American Heart Association (AHA), National Institutes of Health (NIH/NIDDK), and Start-up research funds from the Medical College of Georgia at Augusta University
Research Description
Every 30 minutes, your kidneys filter all the blood in your body, removing waste and excess fluid. 26 million American adults have kidney disease -- and most don't know it. Kidney disease kills over 90,000 Americans every year, which accounts for more deaths than prostate cancer and breast cancer combined (Medical Daily). According to the American Cancer Society, in 2014, approximately 40,000 women died of breast cancer while 29,480 men died of prostate cancer.
When you end up with only one kidney due to developmental problems or surgical renal ablation (secondary to diseased kidney, trauma, or donating a kidney), the nephrons in the remaining kidney will grow larger and consequently the lone kidney gets bigger. In fact, such compensatory renal hypertrophy occurs in virtually all kidney diseases that cause nephron damage and, consequently, a reduction in the number of functioning nephrons. Although the phenomenon of compensatory renal hypertrophy was first described more than a century ago, the underlying signals and mechanisms mediating the onset and extent of compensatory renal hypertrophy have long been a mystery. Our laboratory is interested in identifying the growth signals and molecular signaling mechanisms that regulate the initiation and the extent of compensatory renal hypertrophy. Our initial study revealed that unilateral nephrectomy (UNX) induces compensatory renal hypertrophy of the remaining kidney by activating the mechanistic (originally mammalian) target of rapamycin (mTOR), as documented byour publication in J Am Soc Nephrol. 2005 May;16(5):1384-91. Our follow-up study indicated that homozygous deletion of S6 kinase 1 (S6K1) inhibits renal growth induced by UNX or diabetes (Am J Physiol Renal Physiol. 2009 Sep;297:F585-93). Our further study revealed that phosphorylated ribosomal protein S6 (rpS6) is a major downstream effector of the mTOR complex 1(mTORC1)-S6K1 signaling pathway mediating UNX-induced kidney growth (Kidney Int.2015 Mar;87:543-56). Our continued and focused research in this area has recently led us to identify increased amino acids (delivered into the remaining kidney by increased renal blood flow in response to removal of contralateral kidney) as molecular growth signals that initiate activation of the mTORC1-S6K1 signaling pathway to mediate compensatory renal growth; we have also demonstrated that the signaling pathways of different classes of phosphatidylinositol 3-kinase (PI3K) converge on the activation of mTORC1-S6K1 signaling to regulate kidney size in a context-dependent manner (Figure 1). Thus, our studies have found an answer at the molecular level to explain the century-old question: why does the remaining kidney grow larger when one kidney is lost? For more details, please see the original publication in J Clin Invest.2015 Jun 1;125(6):2429-44 and the related Commentary J Clin Invest. 2015 Jun 1;125:2267-70. Our more recent study revealed striking kidney growth in mice with proximal tubular cell-specific knockout of the tuberous sclerosis 1 gene (Tsc1), which encodes the protein TSC1 (also known as hamartin) as a negative regulator of mTORC1 (Figure 2A). The goal of our continued research endeavor in this area is to identify therapeutic targets to protect nephrons from further injury in order to prevent the development of end-stage renal disease (ESRD), preclude the loss of kidney function, and avoid the painful and costly kidney dialysis and transplantation.
My lab has generated a mammalian homolog of yeast vacuolar protein sorting defective 34 (mVPS34) gene (Pik3c3)-floxed mouse starting from scratch (Figure 2B), as we have recently documented in J Am Soc Nephrol. 2013 Feb;24:198-207. We are now defining the physiological and pathophysiological roles of mVPS34 in the different segments of the nephron in a cell-type specific manner.
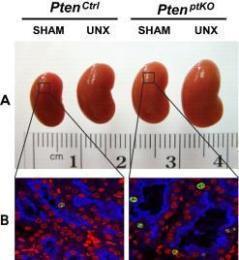
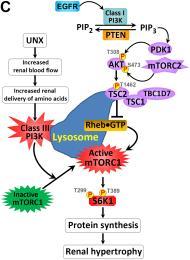
Figure 1: (A-B) Unilateral nephrectomy (UNX) induces equivalent levels of compensatory renal
hypertrophy in proximal tubular cell-specific Pten knockout (PtenptKO) and Pten control(PtenCtrl) mice. Compared with sham-operated PtenptKO mice and PtenCtrl mice, UNX stimulated equivalent
levels of renal hypertrophy in PtenptKO mice and PtenCtrl mice, respectively, as indicated
by increased kidney size (A) and enlarged proximal tubules (B). Note that Pten deletion in renal proximal tubules stimulates slight but statistically
significant cell proliferation, as indicated by increased number of Ki67-positive
cells (green) in the LTA-positive proximal tubules (blue), with DAPI highlighting
all nuclei (red) in the kidney sections. (C) Schematic diagram depicting the regulation of kidney size by PI3K signaling in different
contexts. UNX causes increased renal blood flow (RBF) and therefore increased renal delivery
of free amino acids to the remaining kidney, leading to activation of a PTEN-independent,
but class III PI3K–dependent, mTORC1 translocation to the lysosomal membrane where
the mTORC1 activator RHEB resides and activates mTORC1 signaling to phosphorylate
and activate the downstream effector S6K1. This leads to increased protein synthesis
and renal hypertrophy, while the interplay between PTEN and EGFR-dependent class I
PI3K/mTORC2/AKT/TSC2/mTORC1/S6K1 signaling mediates appropriate kidney weight/BW ratios.
See J Clin Invest. 2015 Jun 1;125(6):2429-44 and J Clin Invest. 2015 Jun 1;125:2267-70 for more details.
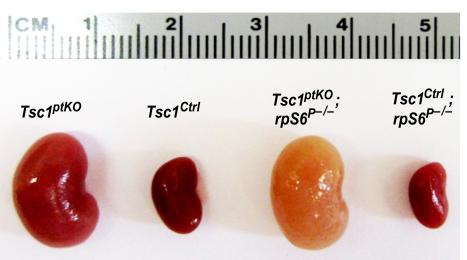
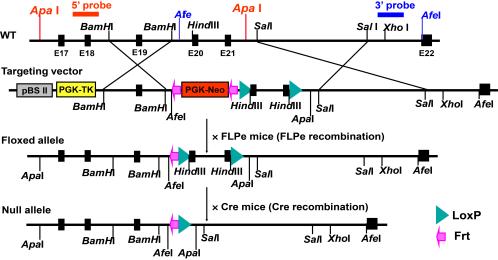
Figure 2: (A) Generation of a new line of proximal tubular cell-specific Tsc1 gene knockout (Tsc1ptKO) mice. These Tsc1ptKO mice exhibit strikingly enlarged kidneys, with minimal cystogenesis
and occasional microscopic tumorigenesis, compared to their wild type control littermates
(Tsc1Ctrl). Knocking non-phosphorylatable ribosomal protein S6 (rpS6P–/–) into the
Tsc1ptKO mice exacerbated cystogenesis and caused drastic nephron damage and renal
fibrosis. These pathological changes caused a pale appearance of the enlarged kidneys.
(B) Creation of an mVPS34 gene (Pik3c3)-floxed mouse. Shown are both the structure of the mVPS34 gene targeting vector and the Cre-LoxP
strategy for creating a conditional mVPS34 knockout mouse. The LoxP sites were inserted
to delete Pik3c3 exons 20-21, which code for the entire catalytic core and the key
DFG motif. The targeting vector was designed to render all the distal exons out of
reading frame so both the catalytic and the ATP binding domains of mVPS34 were deleted.
Selected Publications
1. *Chen JK, Nagai K, Chen J, Plieth D, Hino M, Xu J, Sha F, Ikizler TA, Quarles CC, Threadgill
DW, Neilson EG, and *Harris RC. Phosphatidylinositol 3-kinase signaling determines kidney size. J Clin Invest. 2015; 125(6):2429-44. Epub 2015 May 18. PMID: 25985273.
2014 Impact Factor 13.765
* Corresponding author.
- Featured with a commentary: Al-Awqati Q.“Kidney growth and hypertrophy: the role of mTOR and vesicle trafficking.”>J Clin Invest. 2015 Jun 1;125(6):2267-70. doi: 10.1172/JCI81508. Epub 2015 May 18. PMID: 25985272
- JCI EDITOR’S PICKS, NEPHROLOGY Highlighted in JCI Impact - June 2015 “A signaling pathway that makes kidneys grow”
- Highlighted by Nature Reviews Nephrology: Allison SJ.“Growth and development: Determinants of kidney size” >Nat Rev Nephrol. 2015 Jul; 11(7):387. doi: 10.1038/nrneph.2015.86. Epub 2015 Jun 2. PMID: 26035772
2. Ma SK, Wang Y, Chen J, Zhang MZ, Harris RC, and Chen JK. Overexpression of G-protein-coupled receptor 40 enhances the mitogenic response
to epoxyeicosatrienoic acids. See comment in PubMed Commons below PLoS One. 2015 Feb 13; 10(2):e0113130. doi: 10.1371/journal.pone.0113130. eCollection 2015.
PMCID: PMC4332471.
2014 Impact Factor 3.534
3. Xu J, Chen J, Dong Z, Meyuhas O, and Chen JK. Phosphorylation of ribosomal protein S6 mediates compensatory renal hypertrophy.
Kidney Int. 2015; 87(3):543-56. Epub 2014 Sep 17.
2014 Impact Factor 8.52
4. Chen J, Chen JK, Harris RC. EGF receptor deletion in podocytes attenuates diabetic nephropathy. J Am Soc Nephrol. 2015; 26(5):1115-25. Epub 2014 Sep 3.PMCID: PMC4413759.
2014 Impact Factor 9.466
5. Chen J, Chen MX, Fogo AB, Harris RC, and Chen JK. MVps34 deletion in podocytes causes glomerulosclerosis by disrupting intracellular
vesicle trafficking. J Am Soc Nephrol. 2013 Feb; 24(2):198-207. doi: 10.1681/ASN.2012010101. Epub 2013 Jan 4. PMCID: PMC3559479.
2013 Impact Factor 9.466
6. *Chen JK, Chen J, Thomas G, Kozma SC, and Harris RC. S6 kinase 1 knockout inhibits uninephrectomy- or diabetes-induced renal hypertrophy. Am J Physiol Renal Physiol. 2009; 297(3):F585-93. PMCID: PMC2739710. *Corresponding author.
- Awarded “The 2009 American Journal of Physiology-Renal Physiology Paper of the Year”
- Featured with an editorial commentary: “The growing importance of mTORC1-S6K1 signaling in kidney.” Am J Physiol Renal Physiol. 2009; 297(3):F583-4. Epub 2009 Jul 1.
7. *Chen JK, Chen J, Neilson EG, and Harris RC. Role of mammalian target of rapamycin signaling
in compensatory renal hypertrophy. J Am Soc Nephrol. 2005; 16(5):1384-91. Epub 2005 Mar 23. *Corresponding author.
2005 Impact Factor 7.24
8. Chen JK, Capdevila J, and Harris RC. Heparin-binding EGF-like growth factor mediates the
biological effects of P450 arachidonate epoxygenase metabolites in epithelial cells.
Proc Natl Acad Sci U S A. 2002 Apr 30; 99(9):6029-34. PMCID: PMC122896.
2002 Impact Factor 10.7
9. Chen JK, Falck JR, Capdevila J, and Harris RC. Cytochrome P450 epoxygenase metabolism of
arachidonic acid inhibits apoptosis. Mol Cell Biol. 2001; 21(18):6322-31. PMCID: PMC87364.
2001 Impact Factor 9.836
10. Chen JK, Wang DW, Falck JR, Capdevila J, and Harris RC. Transfection of an active cytochrome
P450 arachidonic acid epoxygenase indicates that 14,15-epoxyeicosatrienoic acid functions
as an intracellular second messenger in response to epidermal growth factor. J Biol Chem. 1999 Feb 19; 274(8):4764-9.
1999 Impact Factor 7.666
11. Chen JK, Falck JR, Reddy KM, Capdevila J, and Harris RC. Epoxyeicosatrienoic acids and their
sulfonamide derivatives stimulate tyrosine phosphorylation and induce mitogenesis
in renal epithelial cells. J Biol Chem. 1998 Oct 30; 273(44):29254-61.PMID: 9786938.
1998 Impact Factor 7.199