
Liu Lab
Current Members

Dr. Kebin Liu, PI

Dafeng Yang
- Lab Manager

Dakota B. Poschel
- MD-PhD Student

Patrick Czabala
- MD-PhD Student

Kathy Li
- MD-Ph.D Student

Kendra Fick
- PhD Student
Zainab Tiamiyu
- PhD Student
Research Program
The research program in the Liu Laboratory focuses on the dynamic interactions between tumor cells and immune cells in the tumor microenvironment. At the molecular level, we aim at elucidating the mechanisms underlying genetic and epigenetic regulation of expression of tumor suppressors and immune checkpoints, including IRF8, Fas, PD-L1, and OPN, in tumor cells and myeloid cells. At the cellular level, we study tumor cell and myeloid cell interactions with the tumor-specific cytotoxic T lymphocytes (CTLs) in the context of CTL immune suppression. Our long-term goal is to develop molecular target-based immunotherapies to enhance human cancer immune surveillance to treat human cancer.
Selected recent publications
- Chunwan Lu, Liyan Liang, Yanmin Wu, Yingcui Yang, Dakota Poschel, Yang Zhao. Lirong Pei, Miao Yu, Martina Zoccheddu, Sergei Bombin, Han-Fei Ding, Huidong Shi, Kebin Liu. 2025. Slc7a5 promotes T cell anti-tumor immunity through sustaining cytotoxic T lymphocyte effector function. Oncogene. In Press.
- Kendra Fick, Nicholas Kerns, Yang Zhao, Zainab Tiamiyu, Dakota B. Poschel, Patrick Czabala, Dafeng Yang, Yi Tang, Jin Xie, Valentyna Fesenkova, Rafal Pacholczyk, Huidong Shi, Kebin Liu, and Priscilla S. Redd. 2025, Lipid nanoparticle-delivered IFNα2 activates Cxcl9 to increase T cell tumor recruitment to suppress lung metastasis. J Immunother Cancer. In Press.
- Dakota B. Poschel, John D. Klement, Alyssa D. Merting, Chunwan Lu, Yang Zhao, Dafeng Yang, Wei Xiao, Huabin Zhu, Ponnala Rajeshwari, Michael Toscano, Kimya Jones, Amanda Barrett, Roni J. Bollag, Padraic G. Fallon, Huidong Shi, and Kebin Liu. 2024. PD-L1 Restrains PD-1+Nrp1lo Treg Cells to Suppress Inflammation-driven Colorectal Tumorigenesis. Cell Rep 43:114819.
- John D Klement, Priscilla S Redd, Chunwan Lu, Alyssa D Merting, Dakota B Poschel, Dafeng Yang, Natasha M Savage, Gang Zhou, David H Munn, Padraic G Fallon, Kebin Liu. 2023. Tumor PD-L1 engages myeloid PD-1 to suppress type I interferon to impair cytotoxic T lymphocyte recruitment. Cancer Cell. 41:620-636.e9.
- Aaron H Colby, Jack Kirsch, Amit N Patwa, Rong Liu, Beth Hollister, William McCulloch, Joanna E Burdette, Cedric J Pearce, Nicholas H Oberliels, Yolonda L Colson, Kebin Liu, Mark W Grinstaff. 2023. Radiolabeled Biodistribution of Expansile Nanoparticles: Intraperitoneal Administration Results in Tumor Specific Accumulation. ACS Nano. 17:2212-2221.
- Zeinab Y. Al Subeh, Dakota B. Poschel, Priscilla S. Redd, John D. Klement, Alyssa D. Merting, Dafeng Yang, Megh Mehta, Huidong Shi, Yolonda L. Colson, Nicholas H. Oberlies, Cedric J. Pearce, Aaron H. Colby, Mark W. Grinstaff, and Kebin Liu. 2022. Lipid nanoparticle delivery of Fas plasmid restores Fas expression to suppress melanoma growth in vivo. ACS Nano. 16:12695-12710.
- Chunwan Lu, Dafeng Yang, John D. Klement, Yolonda L Colson, Nicholas H Oberlies, Cedric J Pearce, Aaron H Colby, Mark W Grinstaff, Zhuoqi Liu, Huidong Shi, Han-Fei Ding, Kebin Liu. 2022. H3K9me3 represses G6PD expression to suppress the pentose phosphate pathway and ROS production to promote human mesothelioma growth. Oncogene. 41:2651-2662.
- Chunwan Lu, Dafeng Yang, Klement, John, Yolonda L. Colson, Nicholas H Oberlies, Cedric J Pearce Aaron H. Colby, Mark W Grinstaff Hanfei Ding, Huidong Shi, and Kebin Liu. 2022. G6PD functions as a metabolic checkpoint to regulate granzyme B expression in tumor-specific cytotoxic T lymphocytes. J Immunother Cancer. 10:e003541. PMCID: PMC8753452.
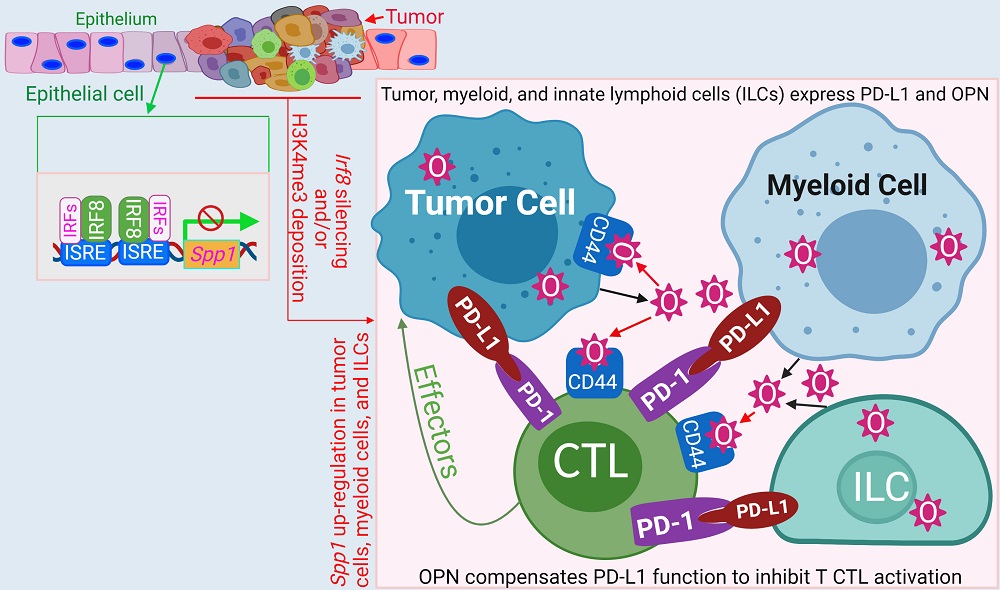
- Chunwan Lu, Zhuoqi Liu, John D Klement, Dafeng Yang, Alyssa D Merting, Dakota Poschel, Thomas Albers, Jennifer L Waller, Huidong Shi, Kebin Liu. 2021. WDR5-H3K4me3 epigenetic axis regulates OPN expression to compensate PD-L1 function to promote pancreatic cancer immune escape. J Immunother Cancer. 9:e002624.
- Huabin Zhu, John D Klement, Chunwan Lu, Priscilla S Redd, Dafeng Yang, Alyssa D Smith, Dakota B Poschel, Juan Zou, Ding Liu, Peng George Wang, David Ostrov, Nicolas Coant, Yusuf A Hannun, Aaron H Colby, Mark W Grinstaff, Kebin Liu. 2021. Asah2 Represses the p53-Hmox1 Axis to Protect Myeloid-Derived Suppressor Cells from Ferroptosis. J Immunol. 206:1395-1404.
- Chunwan Lu, John D Klement, Alyssa D Smith, Dafeng Yang, Jennifer L Waller, Darren D Browning, David H Munn, Kebin Liu. 2020. p50 suppresses cytotoxic T lymphocyte effector function to regulate tumor immune escape and response to immunotherapy. J Immunother Cancer. 8:e001365.
- Alyssa D Smith, Chunwan Lu, Daniela Payne, Amy V Paschall, John D Klement, Priscilla S Redd, Mohammed L Ibrahim, Dafeng Yang, Qimei Han, Zhuoqi Liu, Huidong Shi, Thomas J Hartney, Asha Nayak-Kapoor, Kebin Liu. 2020. Autocrine IL6-mediated Activation of the STAT3-DNMT Axis Silences the TNFα-RIP1 Necroptosis Pathway to Sustain Survival and Accumulation of Myeloid-Derived Suppressor Cells. Cancer Res. 80:3145-3156.
- Christopher Gromisch, Motaz Qadan, Mariana Albuquerque Machado, Kebin Liu, Yolonda Colson, Mark W Grinstaff. 2020. Pancreatic Adenocarcinoma: Unconventional Approaches for an Unconventional Disease. Cancer Res. 80:3179-3192.
- Chunwan Lu, John D. Klement, Wei Xiao, Mohammed Ibrahim, Priscilla S. Redd, Asha Nayak-Kapoor, Gang Zhou, and Kebin Liu. 2019. Type I IFNs activates STAT3 to regulate effector expression of cytotoxic T lymphocyte effectors to suppress tumor development. J Immunother Cancer. 7:157.
- Chunwan Lu, Dafeng Yang, John D. Klement, II Kyu Oh, Natasha M. Savage, Jennifer L. Waller, Aaron H. Colby, Mark W. Grinstaff, Nicholas H. Oberlies, Cedric J. Pearce, Zhiliang Xie, Samuel K. Kulp, Christopher C. Coss, Mitch A. Phelps, Thomas Albers, Iryna O. Lebedyeva, and Kebin Liu. 2019. SUV39H1 represses the expression of cytotoxic T lymphocyte effectors to promote colon tumor immune evasion. Cancer Immunol. Res. 7:414-427.
- John D. Klement, Amy V. Paschall, Priscilla S. Redd, Mohammed L. Ibrahim, Chunwan Lu, Dafeng Yang, Esteban Celis, Scott I. Abrams, Keiko Ozato, and Kebin Liu. 2018. An Osteopontin/CD44 immune checkpoint controls CD8+ T cell activation and tumor immune evasion. J Clin Invest. 128:5549-5560.
- Mohammed L. Ibrahim, John D. Klement, Chunwan Lu, Priscilla S. Redd, Wei Xiao, Dafeng Yang, Darren D. Browning, Natasha M. Savage, Phillip J. Buckhaults, Herbert C. Morse III, and Kebin Liu. 2018. Myeloid-Derived Suppressor Cells Produce IL-10 to Elicit DNMT3b-Dependent IRF8 Silencing to Promote Colitis-Associated Colon Tumorigenesis. Cell Rep. 25:3036-3046.
- Priscilla S. Redd, Mohammed Ibrahim, Sarah K. Sharman, Amy V. Paschall, Dafeng Yang, Asha Nayak-Kapoor, and Kebin Liu. 2017. SETD1B activate iNOS expression in myeloid-derived suppressor cells. Cancer Res. 77:2834-2843.
- Chunwan Lu, Asif Talukder, Natasha Savage, Nagendra Singh and Kebin Liu. 2017. Jak-STAT-mediated chronic inflammation impairs cytotoxic T lymphocyte activation to decrease anti-PD-1 immunotherapy efficacy in pancreatic cancer. OncoImmunology. 6:e1291106.
- Chunwan Lu, Amy V. Paschall, Huidong Shi, Natasha Savage, Jennifer L. Waller, Maria E. Sabbatini, Nicholas H. Oberlies, Cedric Pearce, Kebin Liu. 2017. The MLL1-H3K4me3 Axis-Mediated Up-Regulation of PD-L1 Contributes to Pancreatic Cancer Immune Evasion. J Natl Cancer Inst (JNCI) 109:djw283.